





|

|
|
|

|
Search
03/08/2021
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Don Peacock, DPM, MS
In my 20+ years of practice and surgical correction of first MPJ arthritis, I have learned a lot the hard way. Implants seem to work well at first, but given time, some fail. Fusions work when performed perfectly, although shoe fit and the inability to move the toe bothers many patients. I have done many fusions and many implants; however, I have not done a fusion or implant in about 7 to 8 years since learning MIS techniques.
There is a description of a procedure in minimally invasive foot surgery described by the DePrado which I have used very successfully. I have used the procedure up to stage IV hallux rigidus with success and long-term satisfaction with...
Editor's note: Dr. Peacock's extended-length letter can be read here
Other messages in this thread:
10/29/2021
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Dennis Shavelson DPM, CPed
Podiatry is rapidly losing its iconic ownership of the foot orthotic pie. Isn't it time we stopped marketing foot orthotics that are backed by the same claims that our predecessors made for arch supports in the ‘70s that were backed by anecdotes, not evidence. Dr. Keating's devices do not seem to contact even collapsed feet in optimal functional position (see image).
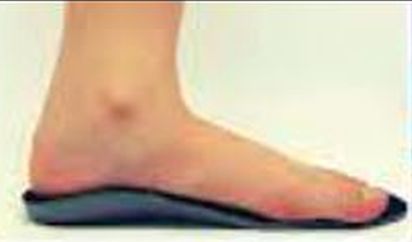 |
Kidzoles |
They lack proven forefoot advances and they do not appear to be able to accomplish many of her claims that the parents of children would pay for in order to prevent injuries, deformities, and performance failures for their kids. Summarily, they appear quite generic.
Dennis Shavelson, DPM, CPed, Tampa, FL
07/12/2021
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Wenjay Sung, DPM
Congrats to Dr. Dana Brems. I hope she serves as an example for all future podiatrists to expand our talents beyond the standard. I'm looking forward to seeing what she does and how the next generation evolves the profession forward.
Wenjay Sung, DPM, Arcadia, CA
03/09/2021
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From Chris Seuferling, DPM
I was wondering if Dr. Peacock could provide video (including x-rays) or a link to the MIS technique for this DePrado procedure. I searched online and on YouTube for one, but only found a short video showing Dr. Peacock with one of his patients post-op clinically.
Chris Seuferling, DPM, Portland, OR
03/05/2021
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Dieter J Fellner, DPM
Congratulations, Dr. Vitale, for adding another implant option to the surgeon's tool box. I am always excited when a new product enters the market, and I have to ask Dr. Vitale: in what way does the Accu-hemi joint provide for 'full joint preservation'? The joint, already damaged from the pathological process of degenerative joint disease, is no longer preserved. Less so, when a set of two reamers are used to prepare the surface for the metal cap (I assume it is metal) that will be placed upon it. Second, I want to ask for the citation of studies that can prove this option is a superior option to existing technology.
Third, I want to ask why the preservation of subchondral bone is such an advance in implant placement. Fourth, in what way does this implant provide for corrected biomechanical function that caused the arthritic damage to the joint? Lastly, we have on the market the Cartiva 'contact lens' implant. This also burns no bridges and, as a patient, I would likely choose that over metal-based alternatives; but that is just my own perspective.
Dieter J Fellner, DPM, NY, NY
05/05/2020
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Bryan C. Markinson, DPM
The FDA approval of Jublia in pediatric patients is a good development. In my practice, 90% of patients interested in possible cure (largely clinical) of onychomycosis are treated with systemic therapy and 90% of those are with oral terbinafine. This includes asymptomatic HIV patients and patients who have been cured of Hep C (in consultation with their hepatologist or ID specialist), and children. Terbinafine in children was off label use, but pediatric dosages have been well established.
Patients are also informed about important adjunctive measures such as cleaning of shoes, avoidance of barefoot walking, treatment of concomitant tinea pedis (including in those they live with), and general good hygiene practices. In 100% of patients in whom I plan to use any FDA approved agent, treatment is NOT started until their diagnosis has been laboratory confirmed. In my hospital laboratory, a PAS stain and fungal culture of deep proximal subungual material results in...
Editor's note: Dr. Markinson's extended-length letter can be read here.
05/04/2020
RESPONSES/COMMENTS (PODIATRIC PRODUCTS AND SERVICES)
From: Keith L. Gurnick, DPM
How wonderful it is that Jublia just got approval from the FDA for use in children age 6 and older. This will be such a helpful tool to those of us who see and treat pediatric patients with onychomycosis. Insurance companies have denied authorization for this safe and effective topical medication based on prior FDA approval in 2014 for age 18 and older. We now have a much better prescription topical medication that can be used safely by pediatric patients with onychomycosis, that insurance companies will not be able to deny based on prior FDA guidelines. They will have to come up with a different reason.
It is sad however, that I first have learned about this from PM News, and not directly from the manufacturer Ortho Dermatologics or my personal Jublia sales rep who could have easily sent me an e-mail during these times when many offices are mostly closed and the drug company sales representatives are not visiting us even if we are open for business. Once Again, I am so thankful that I receive and get to read PM News each day. This service is so informational and helpful to my practice and beneficial for my patients as well.
Keith L. Gurnick, DPM, Los Angeles, CA
|
| |

|
|
|







