02/15/2024
PODIATRIC PRODUCTS IN THE NEWS - PART 2
Waco Shoe Company Unveils Two New Revitalign® Styles
The newest footwear styles by Waco Shoe Company have garnered praise for their health-conscious design without sacrificing the stylistic demands of modern footwear. Waco Shoe Company, a leader in the orthotic footwear market, launched two new shoe designs: Revitalign® Laurel Loafer and Revitalign® Carefree casual shoe.
 |
Dr. Nicholas Pagano |
"The Waco Shoe Company's latest Revitalign® shoes are a leap forward in combining orthotic support with modern style for both men and women. It's impressive to see a brand prioritize foot health without compromising on design," said podiatrist Nicholas Pagano, DPM.
Source: Cision [2/13/24]
02/15/2024
PODIATRIC PRODUCTS IN THE NEWS - PART 1
Balance Doctor's "Balance Sock" Authorized by VA and Defense Dep’t.
Balance Doctor LLC has announced a significant milestone for its flagship product, the Balance Sock, a result of extensive research and innovative design by podiatrist. Mike Hames, DPM, the Balance Sock was recently fully authorized for patient prescription use within both the Veterans Affairs (VA) and Department of Defense (DOD) systems. This endorsement marks a new chapter in supporting the health and operational readiness of America's military personnel.
 |
Dr. Michael Hames |
"We are thrilled that the VA and DOD understand how well our product works and can use it to benefit our active soldiers and veterans," said Dr. Hames, Balance Doctor CEO. "We were recently at a VA medical conference and had resounding acceptance by the physicians in attendance, with orders now going out every day. And we are currently working with the DOD to adopt the Balance Sock as standard issue for our troops, helping reduce ankle injuries and falls."
Source: The Messenger [2/13/24]
02/06/2024
PODIATRIC PRODUCTS IN THE NEWS - PART 2
Intelivation Technologies Announces Commercial Launch Of Hammerdesis™
Intelivation Technologies, an innovative medical device company with a cutting-edge orthopedic portfolio has announced the commercial launch of the Hammerdesis™ Interphalangeal Fusion System in conjunction with their exhibit at the Annual Scientific Meeting of ACFAS.
 |
Dr. Jason Miller |
Jason R. Miller, DPM of Premier Orthopedics remarked, “I am looking forward to the launch of the Hammerdesis™ System. This will not only bring time savings in the OR, but also provide optimal fusion of the joint. The peg and hole procedure has been clinically proven to be very effective, and the Hammerdesis™ is the ideal implant for this procedure.”
Source: Josh Sanberg, OrthoSpineNews [2/1/24]
02/06/2024
PODIATRIC PRODUCTS IN THE NEWS - PART 1
Stryker Expands Prophecy® Surgical Planning System to Include the New Footprint™
Stryker, a global leader in medical technologies, has announced the launch of Prophecy Footprint, an expansion of the Prophecy Surgical Planning system, offering comprehensive surgical planning across the entire foot. The system was demonstrated at the American College of Foot and Ankle Surgeons (ACFAS) Annual Meeting,
 |
Dr. Randy Clements |
The event featured an engaging didactic session titled “Advancements in Surgical Planning for Total Ankle Replacement,” led by Dr. Hodge Davis and Randy Clements, DPM that will explore how Prophecy Footprint provides insights into potential ancillary procedures, contributing to the success of total ankle replacements.
Source: MidFloridaNewspapers.com [1/31/24]
11/03/2023
PODIATRIC PRODUCTS IN THE NEWS - PART 2
FL Podiatrist Joins Forces with Regenative Labs for Brother in Arms Foundation
The Emerald Coast Foot and Ankle Center, led by Carl Speer, DPM answered the call to help local veterans through a donation campaign established by Pensacola-based Regenative Labs (Regenative). Earlier this year, Regenative, a leading HCT/P manufacturer partnered with the Brothers in Arms Foundation (BIAF), a 501(C)(3) non-profit that provides logistical and financial support to wounded, ill, injured, and fallen Marines, sailors, and the families of those who served.
 |
Dr. Carl Speer |
Regenative has donated approximately $5 million dollars of its state-of-the-art Wharton's Jelly product, ProText™, to BIAF which is facilitating the care of qualifying veterans. To bring this care to veterans, Regenative and BIAF needed the help of physicians to apply the product. "When I heard about this opportunity, I immediately wanted to be a part of the effort. Since Pensacola is home to countless veterans, I knew this was a campaign where our center could make a real difference in the quality of peoples' lives," said Dr. Speer.
Source: Cision [11/1/23]
11/03/2023
PODIATRIC PRODUCTS IN THE NEWS - PART 1
Paul Langer Rides in 52nd BikeMS Fundraiser
On October 22, 2023, Paul Langer (McClain Laboratories) rode in his 52nd BikeMS fundraising bicycle tour for the National Multiple Sclerosis Society. The BikeMS route is a thirty mile loop around the perimeter of Manhattan Island. Paul was accompanied by his two sons, family members, and approximately 2,500 other riders. The tour was very successful and has garnered over $1,800,000 this year to date.
 |
(L-R) Paul Langer, Noah Langer, and Fred Langer |
Langer has been participating in this tour since its inception in 1988, sometimes riding in three tours in a year. In addition to New York City, he has ridden in California and Long Island where he was the Volunteer Chairman for eight years. The National Multiple Sclerosis Society funds research for a cure and sponsors support programs for people and families living with multiple sclerosis.
11/09/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 2
Biomedix Provides Aid to Customers Affected by Hurricane Harvey
Hurricane Harvey was the costliest hurricane on record, and displaced over 30,000 people in Texas and Louisiana. Biomedix is committed to helping PADnet users get back on their feet. “Our Customer Service team has already reached out to a number of PADnet customers, offering extensions of service to cover the time they couldn’t perform PADnet studies due to the circumstances surrounding this natural disaster,” shared Biomedix CEO John Romans.
 |
John Romans |
In addition, Biomedix is even offering PADnet users without Biomedix service a period of no-cost service and support. “Practices with PADnet can reach out to Biomedix Customer Service to confirm that they were impacted, and we’ll develop a customized, no-cost program to get their PADnet program up and running,” said Chris Trygstad, Network Director of Biomedix.
11/09/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 1
BAKO Announces New National Sales Manager
BAKO has announced that Kevin Nicholls has joined BAKO, a national leader in podiatric pathology services, as National Sales Manager. Nicholls, a graduate of the University of Michigan’s Ross Business School, has an extensive background in medical sales, including employment at Quest Diagnostics as Sales Director, a 20-year tenure with Pfizer Pharmaceutical, and most recently as Business Director for Myriad Diagnostics’ Assurex Health.
 |
Kevin Nicholls |
Dr. Laurence McCarthy, BAKO CEO states, “Kevin has a passion for his work and is known for his consultative approach to clients and colleagues. After an extensive search, we are honored to have Kevin join the BAKO Leadership Team.”
11/01/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 1
BAKO Announces New CEO
In October, Bako Integrated Physician Solutions celebrated nine years of partnership with the podiatric profession. With 230 employees strong, and a seasoned dermatopathology team, BAKO is a leading provider of diagnostic and therapeutic solutions for the podiatric community across the nation. Over the years, BAKO has evolved into one of the profession’s principal sources of educational support. BAKO has continued to thrive and develop its executive and operations management under the direction of new CEO, Laurence McCarthy, PhD. McCarthy returns to BAKO after serving as BAKO’s Executive Chairman from 2011–2016. In July 2017, Dr. McCarthy served as acting CEO prior to accepting the full-time position in September.
 |
Dr. Laurence McCarthy |
McCarthy states, “True to its vision, BAKO continues to develop new and innovative tests and services for podiatrists and their patients, as well as continuing the company’s extensive support of educational activities within the podiatric community. It continues to be an honor to work with the BAKO team. The professionalism and commitment of our people to the field of podiatry is truly outstanding. We plan to announce further additions to our organization that will reflect our continued dedication to this great profession.”
07/17/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 2
Upstep Launches Online Custom Orthotics Service
Upstep, a company that designs and manufactures orthotics, has launched an online custom orthotics service in the United States. Customers can visit the company’s website to order a footprint kit, which they can use to make their own foot imprint. Upstep makes a 3D scan of the footprint and uses it to design a customized orthotic. Prices for standard orthotics range from $189 to $239.
 |
Phillip Wells |
The company encourages customers to contact their in-house experts, including chief UK podiatrist Phillip Wells, before ordering their orthotics. The orthotics are delivered to the customer’s home by FedEx, along with a series of personalized exercises designed to help the customer improve their foot health. Upstep will adjust the orthotics if the customer is not satisfied, and they offer a full refund within 60 days of purchase.
Source: Leila Meyer, HME Business [7/13/17]
07/17/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 1
NY Podiatrist Uses Clearanail to Treat Onychomycosis
“Nail fungus is not just a cosmetic concern. It can lead to other skin and nail problems, especially in diabetics,” says Mark Friedman, DPM a podiatrist with nearly 20 years of experience in the Capital District. “There are a variety of store-bought topical products, but the success of these methods is limited,” Dr. Friedman explains. “Oral medications, while more effective, are not always an option for patients. Other treatments require removal of the affected toenail, creating an open wound, which is not often advisable.
 |
Dr. Mark Friedman |
Clearanail is a new drug-free, pain-free medical procedure for assisting in the treatment of unsightly and embarrassing toenails. Treatment requires only one appointment, 40 minutes or less – depending on the number of toenails affected. “Controlled micro-perforations are created in the nail allowing for treatment of the contaminated area, leaving the entire nail intact,” Dr. Friedman explains. “There is no pain and only one visit is required. Improvement has been seen in as little as 8-12 weeks.”
Source: Spotlightnews.com [7/14/17]
05/22/2017
PODIATRIC PRODUCTS IN THE NEWS - PART 1
Former NYSPMA ED Appointed as TCP Director of Marketing
Talar Capital Partners (TCP) has announced the appointment of Len Thaler as TCP’s Director of Marketing – East Coast. Prior to joining the TCP team, Mr. Thaler served as the Executive Director (ED) of the New York State Podiatric Medical Association (NYSPMA) from 2004 to 2014. Prior to that, he served as Senior Vice President of Planning and Program Development for ten years at The Jewish Home and Hospital in New York.
 |
Len Thaler |
In addition, Mr. Thaler brings a large network of established relationships with podiatric physicians across the United States. As the Director of Marketing – East Coast, Len will be responsible for increasing TCP membership primarily on the East Coast, in addition to assisting current TCP members in taking full advantage of the savings and benefits of TCP membership.
03/10/2016
PODIATRIC PRODUCTS IN THE NEWS
AMNIOEXCEL® Amniotic Allograft Membrane Study Published in WOUNDS
Derma Sciences, Inc., a tissue regeneration company focused on advanced wound and burn care, has announced the publication of a peer-reviewed comparative clinical study. It compared AMNIOEXCEL® Amniotic Allograft Membrane plus standard-of-care (SOC) with SOC alone for the closure of hard-to-treat diabetic foot ulcers. The results are statistically significant and clinically meaningful, with the closure rate at six weeks in the AMNIOEXCEL® plus SOC treatment arm, far exceeding SOC alone treatment.
The study, “A Prospective, Randomized, Controlled, Multicenter Evaluation of the Use of Dehydrated Amniotic Membrane Allograft Compared to Standard of Care for the Closure of Chronic Diabetic Foot Ulcers,” was published in the peer-reviewed journal WOUNDS. The publication is expected in the March 2016 print issue of the journal. The study was funded by Derma Sciences and the paper was authored by Robert J. Snyder, DPM; Kenneth Shimozaki, DPM; Arthur Tallis, DPM; Michael Kerzner, DPM; Alexander Reyzelman, DPM; Dimitrios Lintzeris, DO; Desmond Bell, DPM; Randi Rutan; and Barry Rosenblum, DPM.
Source: Biospace [3/7/16]
03/08/2016
PODIATRIC PRODUCTS IN THE NEWS
DT Medtech Acquires Hintermann Foot & Ankle Assets
DT MedTech announced that it has acquired the Hintermann lower extremities assets for an undisclosed amount, including the H3 total ankle replacement and revision system and the Kalix II implant designed to treat flat-foot deformities. DT MedTech said it's owned by publishing firm Data Trace Partners and Robeama, a Swiss consulting shop. Dr. David Reicher, a podiatrist, is also chairman and CEO of Data Trace.
"Working diligently alongside Prof. Beat Hintermann, we plan to expand worldwide distribution of the Hintermann Series H3 total ankle replacement and will introduce Prof. Hintermann’s innovative, two-piece total ankle replacement device in the very near future. We want to assure all surgeons who currently use these products that the newly acquired devices will remain exactly the same in structure and materials. Additionally, we foresee no disruption in terms of product availability,” said DT Medtech president & CEO Dr. David Reicher.
Source: Brad Perriello, Mass Device [3/4/16]
02/24/2016
PODIATRIC PRODUCTS IN THE NEWS
Kingetics Spring Lever Orthotic is Finalist in Wearable Robotics Competition
Kingetics, LLC and NDSU were finalists in the innovation challenge with their submission titled “Advanced Composite Spring-Lever Simple Machine for the Enhancement of Reciprocal Gait” at the inaugural meeting of the Wearable Robotics Conference WearRAcon16 sponsored by Intel, Ossur, and IEEE at the University of Arizona.
 |
Dr. Steven King |
Kingetics researcher Dr. Steven King said of the conference, "When you look at the quality of the speakers and the wide range of specialties they represented with the same common goal, you get excited about the intellectual efforts and innovations occurring."
02/22/2016
PODIATRIC PRODUCTS IN THE NEWS
NJ Podiatrist Joins Vionic Innovation Lab
A surgically trained doctor of podiatric medicine specializing in the prevention and treatment of foot pathology, Jacqueline Sutera, DPM joins Vionic Innovation Lab (VIL) to offer preventative foot health solutions to help Americans achieve optimal foot health. Dr. Sutera will join world-renowned leaders in health and lower-limb biomechanics, including pioneer of integrative medicine Andrew Weil MD, podiatric surgeon David Armstrong, DPM, MD, PhD, sports physical therapist Brian Hoke, DPT, celebrity fitness trainer Juliet Kaska, and British podiatric surgeon Trevor Prior.
 |
Dr. Jacqueline Sutera |
Dr. Sutera is a spokesperson for the American Podiatric Medical Association (APMA) and the New York State Podiatric Medical Association. She practices in a private offices in NJ and NYC. She also has surgical affiliations at The Fifth Avenue Surgical Center in Manhattan, and The Metropolitan Surgical Center in Hackensack, NJ.
02/19/2016
PODIATRIC PRODUCTS IN THE NEWS
Neurogenx Opens Second NerveCenter in TN
The second Neurogenx NerveCenter in Tennessee opened Feb. 8 in Jackson, TN. One of only six NerveCenters across the country, the Jackson medical facility is a partnership between David Dowell, DPM, and Neurogenx, the creators of an innovative nerve treatment program that has helped physicians end neuropathy pain for hundreds of patients.
 |
Dr. David Dowell at grand opening of Neurogenx NerveCenter |
NerveCenters are staffed by specially trained clinicians, certified in the Neurogenx treatment protocol. The centers are independently owned and operated, but receive training, marketing, and business support from Neurogenx corporate headquarters. The treatment is non-surgical and non-narcotic. The technology behind the treatment is patented and FDA-cleared.
Source: The Jackson Sun [2/17/16]
02/16/2016
PODIATRIC PRODUCTS IN THE NEWS
TN Podiatrist to Open New Orthotics Facility
Sole Supports’ new facility will create at least 54 jobs and will invest $500,000 in Lewis County. The foot orthotics manufacturer already has a factory in Hickman County and has been located in Tennessee since its inception in 1992. Dr. Edward Glaser founded Sole Supports in 1992 after several years of working as a podiatrist. Originally from New York, he moved to Tennessee because of a cross-country bike ride taken when he was a teenager.
 |
Dr. Edward Glaser |
“He found that Tennessee had the best people. They would invite him into their home,” plant manager Keith Copley said. “He really fell in love with Tennessee, and that’s why he founded the company here.” Glaser set out to produce his own foot supports after realizing the ones he was giving to his patients weren’t effective.
Source: Savanna Walker, columbiadailyherald.com [5/13/16]
02/15/2016
PODIATRIC PRODUCTS IN THE NEWS
20/20 Imaging Showcases A Diagnostic Ultrasound System
20/20 Imaging, a Konica Minolta Company, specialists in imaging and connectivity technologies, showcased SONIMAGE HS1, a cutting-edge diagnostic ultrasound system, at the NY Podiatric Clinical Conference and Exhibition. SONIMAGE HS1 provides the advantages of real time imaging of soft tissue trauma, needle placement visualization, and localization of foreign bodies. “The response to a recent PM News survey revealed that 43% of podiatrists utilize diagnostic ultrasound in their practice”, says 20/20 President, Bob Salzman.
 |
Dr. Kenneth Meisler demonstrates SONIMAGE HS1 |
Besides fostering patient-doctor interaction during the exam, ultrasound-guided needle injections are more effective than blind procedures. The SONIMAGE HS1 provides a Simple Needle Visualization (SNV) feature which enhances the visual presence of the needle tip, increasing accuracy of the needle placement. Also, the SONIMAGE HS1 is compatible with a wide MHz range transducer which provides superior images during both deep and superficial scanning. The SONIMAGE HS1 ultrasound system is supported by comprehensive training, along with tips on assimilating ultrasound into a podiatry practice.
02/11/2016
PODIATRIC PRODUCTS IN THE NEWS
Sensoria Appoints AZ Podiatrist to Scientific Advisory Board
Sensoria Inc., a world leader in the development of smart garments and wearable systems, has appointed Dr. David G. Armstrong to head its newly established Scientific Advisory Board. As a leader in podiatric surgery and research, Dr. Armstrong holds the honor of being the youngest ever recipient of the American Diabetes Associations’ (ADA) Roger E. Pecoraro Award—the highest award given in his field—and is the youngest-ever member to be elected into the PM Podiatry Hall of Fame.
 |
Dr. David Armstrong |
In his new role on Sensoria’s Scientific Advisory Board, Dr. Armstrong will focus on the scientific validation of Sensoria’s technology in clinical applications. “Textile sensing infused smart garments have the potential to be a game-changer in managing and measuring how we move through the world,” said Dr. Armstrong, a tenured Professor of Surgery at The University of Arizona.
Source: Nasdaq Globenewswire [2/10/16]
02/10/2016
PODIATRIC PRODUCTS IN THE NEWS
Smart Socks Alert Diabetics When Feet are at Risk for Ulcers
Researchers at The Hebrew University of Jerusalem and their colleagues have developed a machine-washable, pressure sensitive sock that can help patients with diabetic neuropathy tell when they are putting their feet at risk for ulcers.
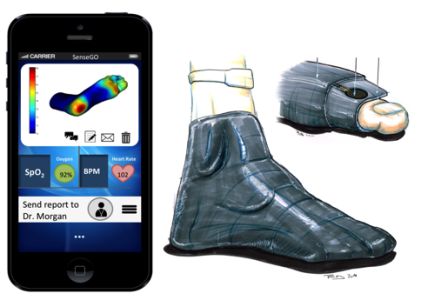 |
SenseGO sock |
The SenseGO sock contains micro-fabricated pressure sensors that can register when inappropriate pressure is put on a diabetic patient’s foot and relays that pressure data to a connected smartphone app, which can then alert the patient to attend to his or her foot and alleviate the pressure.
Source: Univadis [2/4/16]
02/01/2016
PODIATRIC PRODUCTS IN THE NEWS
APMA Collaborates with MedXpress Registry
The American Podiatric Medical Association (APMA) in collaboration with MedXpress Registry Div. of ICS Software, Ltd., have announced the formation of a specialized registry for podiatry. Discounted pricing is available to APMA members. The minimal fee also includes the cost to submit 9 PQRS Measures for 2016 or the PQRS Diabetes Measures Group for 2016. It is important to remember that in order to submit to any specialized registry, DPMs MUST register with that registry by February 29, 2016 (the first 60 days of the reporting period) if they plan on performing Meaningful Use for 2016.
Those who are NOT attempting to meet the requirements for Meaningful Use for 2016 but are reporting PQRS measures for 2016, can register after February 29, 2016, but for Meaningful Use, they must register with a specialty registry by the deadline. Reporting/performing PQRS has nothing to do with the specialty registry. The purpose of the specialty registry is 1) to meet the Meaningful Use measure and 2) to help gather specific data for APMA.
01/28/2016
PODIATRIC PRODUCTS IN THE NEWS
PICA Announces Collaboration with Meet the Masters
PICA, a Franklin, TN based professional liability insurer for podiatric physicians, has entered into a new collaboration with Podiatric Success, home of Meet the Masters. “One of the strengths of PICA is that we are constantly looking for ways to increase our communications with podiatric physicians,” states Dr. Ross Taubman, President of PICA. “The greatest type of communication is interactive. That is why we have decided to build a discussion forum with Meet the Masters, as well as providing topics on their new Lunch with the Masters program. It allows us to address the risk management and practice management concerns of podiatrists head-on,” said Taubman. This new program is projected to roll out by April 1, 2016.
 |
Drs. Ross Taubman and Bret Ribotsky |
With over eight years of history and more than 350 Masters interviewed, the Meet the Masters series takes an in-depth look at the newest treatments and protocols currently available, including information about how to maintain a successful podiatric practice and the most up-to-date news of the trade. “PICA and Meet the Masters are beacons of success in our profession, and this collaboration is a new, professional outlet for today’s busy podiatric physicians to discuss industry, practice, and risk issues,” said Dr. Bret Ribotsky, host of Meet the Masters.
01/25/2016
PODIATRIC PRODUCTS IN THE NEWS
PRESENT e-Learning Systems Announces Alliance Partnership with AAPPM
PRESENT e-Learning Systems has announced its latest Alliance Partner, the American Academy of Podiatric Practice Management (AAPPM). The PRESENT Alliance Partners program establishes cooperative partnerships with membership organizations within the fields of podiatry and wound care, with the goal of supporting each other’s activities and bringing a richer menu of valuable services to each organization’s members. PRESENT e-Learning Systems CEO Alan Sherman, DPM, CCMEP announced the new partnership and said, “We have long admired the valuable contribution made by the American Academy of Podiatric Practice Management (AAPPM) to the management skill set of practicing podiatrists."
 |
Drs. Alan Sherman and Charlie Greiner |
AAPPM President Charlie Greiner, DPM, added, “The AAPPM exists to share knowledge, ideas, and the tools that all podiatrists in practice need to be successful practitioners. We are thrilled to become a part of the PRESENT e-Learning Systems, and we are excited about the opportunities to educate an even larger network of colleagues and staff.” PRESENT e-Learning System’s Alliance Partners include the American Board of Podiatric Medicine, American Board of Multiple Specialties in Podiatry, the American Association of Women Podiatrists, the American Academy of Podiatric Sports Medicine, the Canadian Federation of Podiatric Medicine, and the American College of Foot and Ankle Pediatrics.
01/21/2016
PODIATRIC PRODUCTS IN THE NEWS
MI Podiatry Clinic Steps Into RFID for Tool Tracking
Great Lakes Foot and Ankle Specialists has automated the tracking of surgical instruments or tools used on its patients via radio frequency identification, in order to speed up the process of packing the tools onto trays, ensure that no instruments are left inside a patient or misplaced, and track their lifespan. The Surgical Safety Scanner system, provided by startup Surgical Scanner, of Brighton, MI, consists of passive ultrahigh-frequency (UHF) tags, handheld readers and software to manage the collected read data. The software also links each tag ID number with a particular instrument's photos, manufacturer, part number, and history.
 |
Dr. Jeff Szczepanski (photo: Tessa Lightly) |
The solution enables the clinic to overcome several challenges, says Jeff Szczepanski, DPM, a physician at the facility. Each surgery requires a specific set of tools, but it can be difficult to differentiate between one tool-packed tray and another. Many of the instruments look alike, so the wrong item could end up on a tray unless each tray is double- or triple-checked during the manual packing process. Once a packed tray is wrapped following sterilization, it can also be difficult to determine and confirm which tools are on it. As a result, the wrong tray could be taken to surgery. Additionally, Szczepanski says, the items are expensive (some priced at $300 to $400) and can end up missing. When this occurs, he adds, tracing back where a particular instrument was lost is often impossible.
Source: Claire Swedberg, RFID Journal [1/18/16]







